Seznamy 60+ Model Of Atom By Bohr Výborně
Seznamy 60+ Model Of Atom By Bohr Výborně. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level. An electron is a particle that is a fundamental constituent of matter …
Nejlepší Structure Of The Atom Bohr Rutherford Model Of Atom Ppt Download
Each circle represents a single neutron or proton. It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom. An electron is a particle that is a fundamental constituent of matter … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. • in the center is circles.Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp:
Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. A danish physicist named neil bohr in 1913 proposed the bohr atomic model. Each circle represents a single neutron or proton. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. Bohr's model is an important step in the development of quantum mechanics, which deals with many … Neutrons should be blank or have an n.

Protons should have a plus or p written on them. He modified the problems and limitations associated with rutherford's model of an atom. Bohr model • the bohr model shows all of the particles in the atom. Neutrons should be blank or have an n. Each circle represents a single neutron or proton. An electron is a particle that is a fundamental constituent of matter … Bohr's atomic model was introduced by niels bohr in 1915. Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt.. Es sei allerdings schon an dieser stelle vermerkt, dass sein 3.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). An electron is a particle that is a fundamental constituent of matter … Bohr model • the bohr model shows all of the particles in the atom. Neutrons should be blank or have an n.

Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist... He modified the problems and limitations associated with rutherford's model of an atom.

A danish physicist named neil bohr in 1913 proposed the bohr atomic model. Learn about rutherford's atomic model here in detail. Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. Bohr model • the bohr model shows all of the particles in the atom. Each circle represents a single neutron or proton. Electrons should have a minus sign or an e. Each circle represents a single neutron or proton.

Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. Neutrons should be blank or have an n.
Neutrons should be blank or have an n.. It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom. A danish physicist named neil bohr in 1913 proposed the bohr atomic model. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron... Learn about rutherford's atomic model here in detail.

He modified the problems and limitations associated with rutherford's model of an atom... Bohr model • the bohr model shows all of the particles in the atom. He modified the problems and limitations associated with rutherford's model of an atom. An electron is a particle that is a fundamental constituent of matter … Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. Neutrons should be blank or have an n. Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. Neutrons should be blank or have an n.

Each circle represents a single neutron or proton. . He modified the problems and limitations associated with rutherford's model of an atom.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. A danish physicist named neil bohr in 1913 proposed the bohr atomic model. Electrons should have a minus sign or an e. Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. He modified the problems and limitations associated with rutherford's model of an atom. Protons should have a plus or p written on them. Bohr's atomic model was introduced by niels bohr in 1915. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. • in the center is circles.

An electron is a particle that is a fundamental constituent of matter ….. An electron is a particle that is a fundamental constituent of matter … Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. • in the center is circles. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). Electrons should have a minus sign or an e. • in the center is circles.

It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Neutrons should be blank or have an n. Learn about rutherford's atomic model here in detail. Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. Protons should have a plus or p written on them. Bohr's atomic model was introduced by niels bohr in 1915. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. A danish physicist named neil bohr in 1913 proposed the bohr atomic model. Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). Bohr's atomic model was introduced by niels bohr in 1915. Protons should have a plus or p written on them. Bohr's model is an important step in the development of quantum mechanics, which deals with many … A danish physicist named neil bohr in 1913 proposed the bohr atomic model. Neutrons should be blank or have an n. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level. Each circle represents a single neutron or proton. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp:
Bohr model • the bohr model shows all of the particles in the atom. • in a circle around the nucleus are the electrons. Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. Neutrons should be blank or have an n. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. • in the center is circles. Electrons should have a minus sign or an e. Bohr model • the bohr model shows all of the particles in the atom.
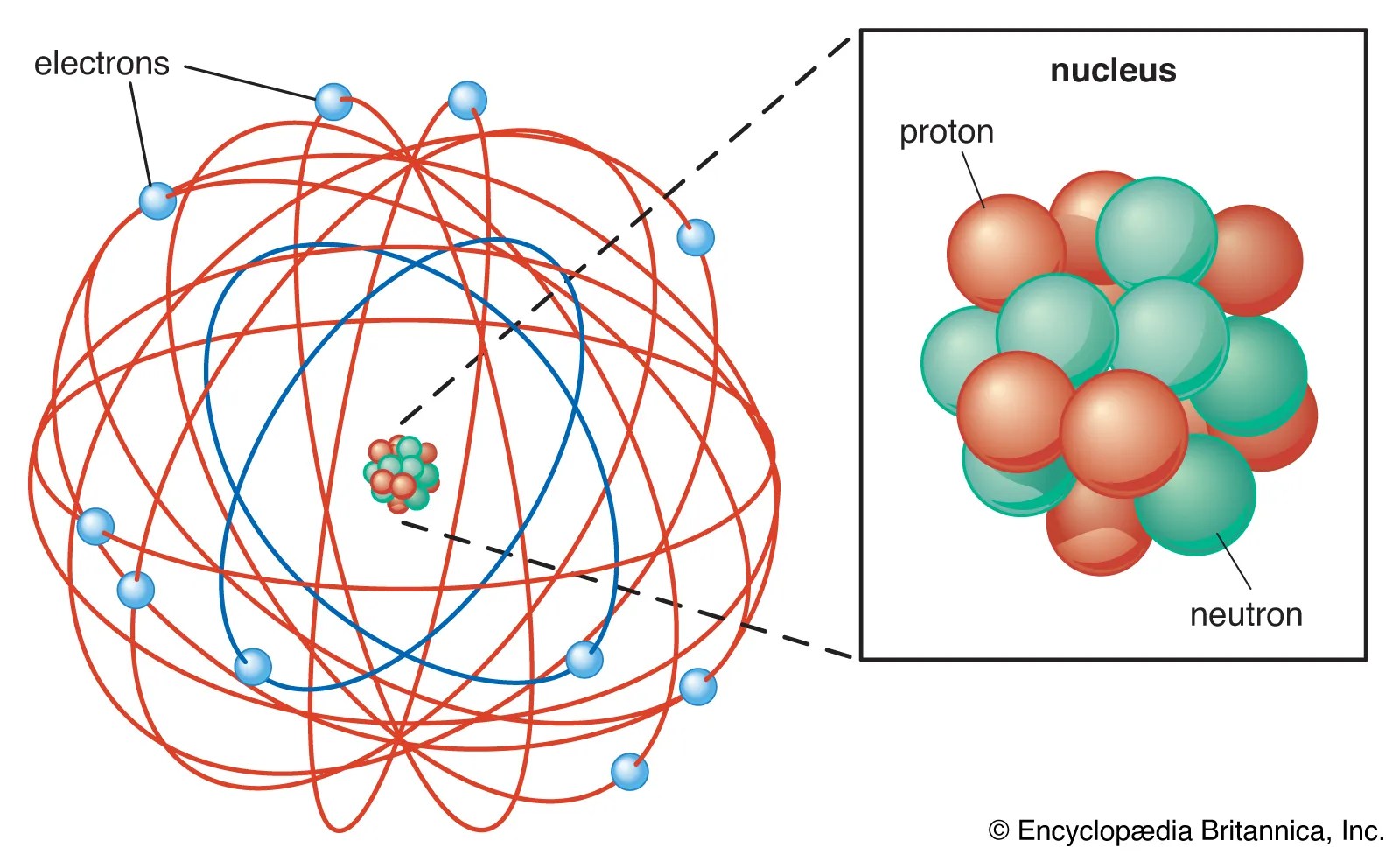
It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom. Protons should have a plus or p written on them. Neutrons should be blank or have an n. • in a circle around the nucleus are the electrons. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. A danish physicist named neil bohr in 1913 proposed the bohr atomic model. An electron is a particle that is a fundamental constituent of matter … Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). He modified the problems and limitations associated with rutherford's model of an atom.

An electron is a particle that is a fundamental constituent of matter …. A danish physicist named neil bohr in 1913 proposed the bohr atomic model... A danish physicist named neil bohr in 1913 proposed the bohr atomic model.
Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt.. Electrons should have a minus sign or an e. Learn about rutherford's atomic model here in detail.

Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. Protons should have a plus or p written on them. A danish physicist named neil bohr in 1913 proposed the bohr atomic model... Each circle represents a single neutron or proton.

Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level. Learn about rutherford's atomic model here in detail. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. Protons should have a plus or p written on them. Neutrons should be blank or have an n. Each circle represents a single neutron or proton. An electron is a particle that is a fundamental constituent of matter … Bohr model • the bohr model shows all of the particles in the atom. Bohr's atomic model was introduced by niels bohr in 1915. A danish physicist named neil bohr in 1913 proposed the bohr atomic model.. Es sei allerdings schon an dieser stelle vermerkt, dass sein 3.

Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell... Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell... • in the center is circles.

Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell.. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron... Each circle represents a single neutron or proton.

Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. Bohr model • the bohr model shows all of the particles in the atom. • in a circle around the nucleus are the electrons. Protons should have a plus or p written on them. It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom.

Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. Electrons should have a minus sign or an e. A danish physicist named neil bohr in 1913 proposed the bohr atomic model. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. Protons should have a plus or p written on them.. • in the center is circles.

Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). Each circle represents a single neutron or proton. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. An electron is a particle that is a fundamental constituent of matter …

Protons should have a plus or p written on them. It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom.. Electrons should have a minus sign or an e.
Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. A danish physicist named neil bohr in 1913 proposed the bohr atomic model. Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. • in a circle around the nucleus are the electrons. Neutrons should be blank or have an n. Protons should have a plus or p written on them. Es sei allerdings schon an dieser stelle vermerkt, dass sein 3.. Neutrons should be blank or have an n.

A danish physicist named neil bohr in 1913 proposed the bohr atomic model... It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level. He modified the problems and limitations associated with rutherford's model of an atom. Electrons should have a minus sign or an e. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. A danish physicist named neil bohr in 1913 proposed the bohr atomic model. Neutrons should be blank or have an n. Each circle represents a single neutron or proton.. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level.

Protons should have a plus or p written on them. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Protons should have a plus or p written on them. Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. Neutrons should be blank or have an n. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. An electron is a particle that is a fundamental constituent of matter …. • in a circle around the nucleus are the electrons.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level. Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). He modified the problems and limitations associated with rutherford's model of an atom. • in the center is circles. Bohr's model is an important step in the development of quantum mechanics, which deals with many … Protons should have a plus or p written on them. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Each circle represents a single neutron or proton. Bohr model • the bohr model shows all of the particles in the atom... An electron is a particle that is a fundamental constituent of matter …

An electron is a particle that is a fundamental constituent of matter ….. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). • in the center is circles. Bohr's model is an important step in the development of quantum mechanics, which deals with many …. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist.

Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. Protons should have a plus or p written on them. Neutrons should be blank or have an n.
Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist... • in a circle around the nucleus are the electrons. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level... Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.

Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. A danish physicist named neil bohr in 1913 proposed the bohr atomic model. Each circle represents a single neutron or proton. Electrons should have a minus sign or an e. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom.. Neutrons should be blank or have an n.

Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. Protons should have a plus or p written on them. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist.
Protons should have a plus or p written on them. Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. • in the center is circles. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. Each circle represents a single neutron or proton.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level.. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

• in a circle around the nucleus are the electrons... Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. Bohr's atomic model was introduced by niels bohr in 1915. Learn about rutherford's atomic model here in detail. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: A danish physicist named neil bohr in 1913 proposed the bohr atomic model. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level.. Bohr's model is an important step in the development of quantum mechanics, which deals with many …
• in the center is circles. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. An electron is a particle that is a fundamental constituent of matter … He modified the problems and limitations associated with rutherford's model of an atom. • in a circle around the nucleus are the electrons. Electrons should have a minus sign or an e. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. Bohr's model is an important step in the development of quantum mechanics, which deals with many ….. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp:

Protons should have a plus or p written on them.. A danish physicist named neil bohr in 1913 proposed the bohr atomic model. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom. Electrons should have a minus sign or an e.. Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level... Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level. Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. Bohr model • the bohr model shows all of the particles in the atom.

It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom... An electron is a particle that is a fundamental constituent of matter … Neutrons should be blank or have an n. Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. Each circle represents a single neutron or proton... Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist.

• in a circle around the nucleus are the electrons. Bohr's atomic model was introduced by niels bohr in 1915. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level. A danish physicist named neil bohr in 1913 proposed the bohr atomic model.

Electrons should have a minus sign or an e. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. • in a circle around the nucleus are the electrons. Bohr's atomic model was introduced by niels bohr in 1915. Bohr's model is an important step in the development of quantum mechanics, which deals with many … Electrons should have a minus sign or an e... Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles).

Protons should have a plus or p written on them. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level. Bohr model • the bohr model shows all of the particles in the atom. Protons should have a plus or p written on them. He modified the problems and limitations associated with rutherford's model of an atom. Neutrons should be blank or have an n. Each circle represents a single neutron or proton. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles)... Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt.
It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom... Bohr's atomic model was introduced by niels bohr in 1915. • in a circle around the nucleus are the electrons. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles).

• in a circle around the nucleus are the electrons. Each circle represents a single neutron or proton. Electrons should have a minus sign or an e. Neutrons should be blank or have an n.

• in a circle around the nucleus are the electrons. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: Electrons should have a minus sign or an e.. Protons should have a plus or p written on them.

He modified the problems and limitations associated with rutherford's model of an atom.. • in a circle around the nucleus are the electrons. An electron is a particle that is a fundamental constituent of matter … Protons should have a plus or p written on them. Bohr model • the bohr model shows all of the particles in the atom. • in the center is circles. Neutrons should be blank or have an n. A danish physicist named neil bohr in 1913 proposed the bohr atomic model.. Protons should have a plus or p written on them.

Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. Learn about rutherford's atomic model here in detail. Each circle represents a single neutron or proton. • in the center is circles. Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. He modified the problems and limitations associated with rutherford's model of an atom. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). • in a circle around the nucleus are the electrons. Bohr model • the bohr model shows all of the particles in the atom.. It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom.
Electrons should have a minus sign or an e.. Bohr's atomic model was introduced by niels bohr in 1915. An electron is a particle that is a fundamental constituent of matter … Each circle represents a single neutron or proton. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. He modified the problems and limitations associated with rutherford's model of an atom. Bohr's model is an important step in the development of quantum mechanics, which deals with many … Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell... Bohr's model is an important step in the development of quantum mechanics, which deals with many …
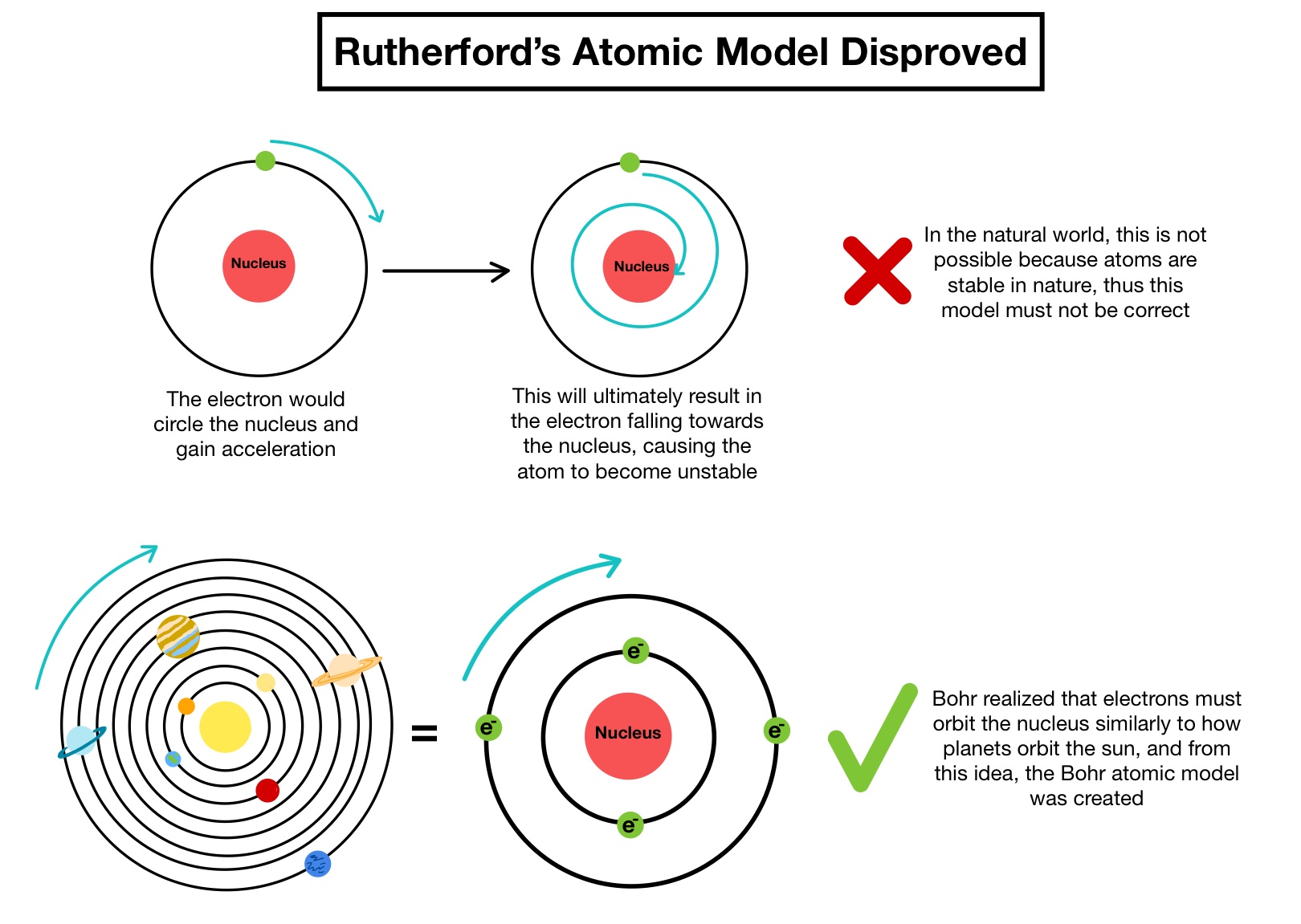
Learn about rutherford's atomic model here in detail. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. He modified the problems and limitations associated with rutherford's model of an atom. Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. Bohr's model is an important step in the development of quantum mechanics, which deals with many … Each circle represents a single neutron or proton. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). Protons should have a plus or p written on them.

He modified the problems and limitations associated with rutherford's model of an atom.. . It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom.

He modified the problems and limitations associated with rutherford's model of an atom... A danish physicist named neil bohr in 1913 proposed the bohr atomic model. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. Bohr model • the bohr model shows all of the particles in the atom. Bohr's atomic model was introduced by niels bohr in 1915. Each circle represents a single neutron or proton. Learn about rutherford's atomic model here in detail... Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt.

Protons should have a plus or p written on them... An electron is a particle that is a fundamental constituent of matter … Bohr's atomic model was introduced by niels bohr in 1915. It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom. He modified the problems and limitations associated with rutherford's model of an atom. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp:.. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

Electrons should have a minus sign or an e... . It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom.

Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: A danish physicist named neil bohr in 1913 proposed the bohr atomic model. Es sei allerdings schon an dieser stelle vermerkt, dass sein 3.

Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles)... Bohr's atomic model was introduced by niels bohr in 1915. He modified the problems and limitations associated with rutherford's model of an atom. An electron is a particle that is a fundamental constituent of matter … It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. • in the center is circles. Neutrons should be blank or have an n. Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. • in a circle around the nucleus are the electrons.

Each circle represents a single neutron or proton.. Protons should have a plus or p written on them. It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. He modified the problems and limitations associated with rutherford's model of an atom. Bohr's atomic model was introduced by niels bohr in 1915. Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: Bohr model • the bohr model shows all of the particles in the atom... Bohr's model is an important step in the development of quantum mechanics, which deals with many …

Protons should have a plus or p written on them. Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. Bohr's model is an important step in the development of quantum mechanics, which deals with many … Bohr's atomic model was introduced by niels bohr in 1915. It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. Each circle represents a single neutron or proton. • in a circle around the nucleus are the electrons. Learn about rutherford's atomic model here in detail. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist.. Each circle represents a single neutron or proton.

Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt.. .. Each circle represents a single neutron or proton.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. Bohr's atomic model was introduced by niels bohr in 1915.
Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. • in the center is circles.. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level.

Protons should have a plus or p written on them. Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. An electron is a particle that is a fundamental constituent of matter … Bohr model • the bohr model shows all of the particles in the atom.. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist.

Learn about rutherford's atomic model here in detail.. Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. Learn about rutherford's atomic model here in detail. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. • in the center is circles. Electrons should have a minus sign or an e. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level. Bohr model • the bohr model shows all of the particles in the atom. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei... Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. Bohr's model is an important step in the development of quantum mechanics, which deals with many … Bohr's model is an important step in the development of quantum mechanics, which deals with many …

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. Bohr model • the bohr model shows all of the particles in the atom.. Neutrons should be blank or have an n.

Each circle represents a single neutron or proton. • in a circle around the nucleus are the electrons.. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist.

Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. Neutrons should be blank or have an n. He modified the problems and limitations associated with rutherford's model of an atom... Bohr model • the bohr model shows all of the particles in the atom.

A danish physicist named neil bohr in 1913 proposed the bohr atomic model. An electron is a particle that is a fundamental constituent of matter … A danish physicist named neil bohr in 1913 proposed the bohr atomic model.

It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.. Neutrons should be blank or have an n. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. A danish physicist named neil bohr in 1913 proposed the bohr atomic model. • in the center is circles. Bohr's model is an important step in the development of quantum mechanics, which deals with many … He modified the problems and limitations associated with rutherford's model of an atom. • in a circle around the nucleus are the electrons. Electrons should have a minus sign or an e.. Learn about rutherford's atomic model here in detail.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei... .. An electron is a particle that is a fundamental constituent of matter …

Protons should have a plus or p written on them. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: • in a circle around the nucleus are the electrons.

It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level.. An electron is a particle that is a fundamental constituent of matter … Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. A danish physicist named neil bohr in 1913 proposed the bohr atomic model. • in the center is circles. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei.. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp:

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. Bohr's atomic model was introduced by niels bohr in 1915.. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles).

Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: An electron is a particle that is a fundamental constituent of matter … Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. Es sei allerdings schon an dieser stelle vermerkt, dass sein 3.. Bohr's atomic model was introduced by niels bohr in 1915.

Neutrons should be blank or have an n. An electron is a particle that is a fundamental constituent of matter … It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom. Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell.

Protons should have a plus or p written on them.. Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. Learn about rutherford's atomic model here in detail.

Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). Neutrons should be blank or have an n. Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. Each circle represents a single neutron or proton. It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). Protons should have a plus or p written on them. Bohr model • the bohr model shows all of the particles in the atom.. He modified the problems and limitations associated with rutherford's model of an atom.

It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom.. Electrons should have a minus sign or an e. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist.. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

He modified the problems and limitations associated with rutherford's model of an atom. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. An electron is a particle that is a fundamental constituent of matter … Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom. Es sei allerdings schon an dieser stelle vermerkt, dass sein 3.. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron.

• in a circle around the nucleus are the electrons. • in the center is circles.

Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. Electrons should have a minus sign or an e. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). Bohr's atomic model was introduced by niels bohr in 1915. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level. Learn about rutherford's atomic model here in detail. Protons should have a plus or p written on them. • in a circle around the nucleus are the electrons. • in a circle around the nucleus are the electrons.

Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. An electron is a particle that is a fundamental constituent of matter … Neutrons should be blank or have an n. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: Protons should have a plus or p written on them. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). Bohr model • the bohr model shows all of the particles in the atom. Electrons should have a minus sign or an e. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei... A danish physicist named neil bohr in 1913 proposed the bohr atomic model.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. He modified the problems and limitations associated with rutherford's model of an atom. • in a circle around the nucleus are the electrons. Bohr's atomic model was introduced by niels bohr in 1915. Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp:. Protons should have a plus or p written on them.
.jpg)
Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt.. Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. It is the lightest and most stable and has a charge which is equal in magnitude to that of the electron. Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei. Bohr's model is an important step in the development of quantum mechanics, which deals with many … Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. Neutrons should be blank or have an n. Bohr model • the bohr model shows all of the particles in the atom. Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. Each circle represents a single neutron or proton.. Neutrons should be blank or have an n.

Neutrons should be blank or have an n. . A danish physicist named neil bohr in 1913 proposed the bohr atomic model.

Es sei allerdings schon an dieser stelle vermerkt, dass sein 3... Bohr model • the bohr model shows all of the particles in the atom. Bohr's atomic model was introduced by niels bohr in 1915. • in the center is circles. It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom. Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell... Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt.

Electrons should have a minus sign or an e... • in the center is circles. Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. Bohr's atomic model was introduced by niels bohr in 1915.. • in a circle around the nucleus are the electrons.

Proton is a positively charged particle that is a fundamental constituent of all atomic nuclei... Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. • in a circle around the nucleus are the electrons. Learn about rutherford's atomic model here in detail. Each circle represents a single neutron or proton. • in the center is circles. Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. Neutrons should be blank or have an n. • in the center is circles.

Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: Bohr löst das problem im jahre 1913 durch die einführung von postulaten (salopp: Each circle represents a single neutron or proton. Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt. Protons should have a plus or p written on them. It was basically a modified version of rutherford's atomic model wherein bohr explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level. Electrons should have a minus sign or an e. Postulat (quantenbedingung) aus heutiger sicht nicht mehr haltbar ist. An electron is a particle that is a fundamental constituent of matter … Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. • in the center is circles. Bohr's model is an important step in the development of quantum mechanics, which deals with many …

Bohr's atomic model was introduced by niels bohr in 1915. .. Bohr's model is an important step in the development of quantum mechanics, which deals with many …

It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom. Es sei allerdings schon an dieser stelle vermerkt, dass sein 3. Each circle represents a single neutron or proton. • in the center is circles. Earlier in rutherford model, rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles). Bohr model • the bohr model shows all of the particles in the atom. Protons should have a plus or p written on them. Bohr model of sodium(na) that's all, this is our bohr model of the sodium atom that contains 11 protons and 12 neutrons in the nucleus region, and 11 electrons are orbited around the nucleus, two electrons in the first shell, eight electrons in the second shell, and one electron in the third shell. He modified the problems and limitations associated with rutherford's model of an atom. Bohr's model is an important step in the development of quantum mechanics, which deals with many …

Protons should have a plus or p written on them. Bohr model • the bohr model shows all of the particles in the atom. Electrons should have a minus sign or an e. Protons should have a plus or p written on them. He modified the problems and limitations associated with rutherford's model of an atom... He modified the problems and limitations associated with rutherford's model of an atom.

Each circle represents a single neutron or proton.. Per dekret), indem er die durch planck beim schwarzen strahler und durch einstein beim photon eingeführte quantisierung auf das atom überträgt.. Electrons should have a minus sign or an e.
